PTFE (polytetrafluoroethylene) is a fluoropolymer and is the most widely recognized term for non-stick or low friction coatings. However, there are two other very important fluoropolymers that are equally important and also form part of our portfolio of coating systems. These are PFA (perfluoroalkoxy) and FEP (fluorinated ethylene propylene). Both have unique properties and there are applications where the use of PFA or FEP based coating systems would be more suitable than a PTFE based system.
It is important to understand that low friction is not the same as non-stick. A coating that provides good low friction characteristics would not necessarily be a good release coating and vice-versa.
Friction is the force generated by two surfaces moving against each other and there are two distinct types of friction that apply to coatings technology. The first is static friction. This is the resistance to initial movement between two surfaces that are in contact. The second is dynamic friction which is the resistance to continued movement between two surfaces that are in contact. As PTFE has an extremely low coefficient of friction, it is the ideal solution where friction causes problems.
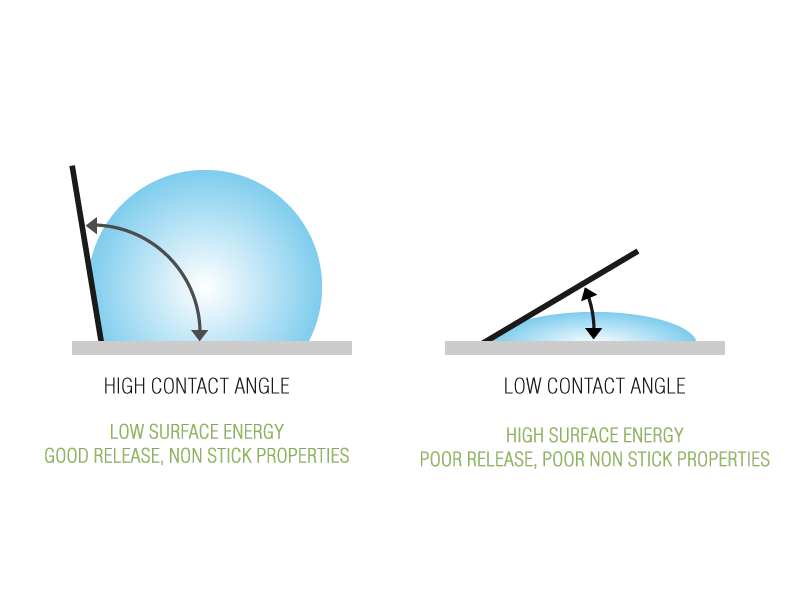
Non-stick or release is a function of low surface energy or the tendency of a surface to repel. This value can be measured by determining the contact angle of a droplet of liquid on a coated surface, the steeper the angle between the contact plane and the bead of water, the lower the surface energy and therefore the greater release properties of the surface. A low surface energy value is a reliable indication of good non-stick or release properties.
We generally associate non-stick or release with the coating on cookware which helps to release food during and after the cooking process. However, release is also a very important factor in industrial processes such as rubber moulding, thermoforming, injection moulding, photocopying and printing machines, adhesive dispensers or assemblies. Good release is also of great value in processes where the build up of foreign particles such as dust, soot, food particles or even ice can clog or jam machinery and processing equipment.
A commonly asked question is how do you make a non-stick coating stick? One of the main factors affecting substrate adhesion is how the surface is prepared.
Almost any foreign substance that is present on the surface of a part is likely to affect the adhesion of the coating. This includes cutting fluids and coolants, moisture, atmospheric dust, corrosion inhibitors and even invisible oxidisation caused by exposure to normal ambient conditions.
Firstly, we have to remove any oils or surfactants that are present. This can be achieved either by using solvents that will remove the contamination or by thermally de-greasing the part, which involves carbonising any foreign substance on the surface.
After the parts have been cleaned or thermally de-greased, the next stage is to grit blast using aluminium oxide with a particle size that is most suited to the substrate type and the type of coating system that has been selected. Gritblasting not only acts as a cleaning process but it also provides a mechanical key for the coating to adhere to by increasing the surface area and roughening the surface of the part. Most coatings benefit from a surface roughness average (Ra) of around 1 – 6 microns, depending on the type of coating system.
Other pre-treatments include acid or alkali etching, pickling or phosphating, depending on the particular substrate material.

PTFE was discovered in 1938 by Roy Plunkett. The discovery was made by accident whilst Plunkett was attempting to make a new chlorofluorocarbon refrigerant. He discovered a residue of white waxy material that was unusually slippery. This was the birth of PTFE, the substance was patented in 1941 and the Teflon trademark was registered in 1945.
Following a period of testing, it became clear the PTFE was an extraordinary substance. Its surface was found to be so slippery that almost nothing would stick to it. Also, it was unaffected by almost every chemical and solvent, moisture had no effect and following exposure to prolonged periods of sunlight, it did not degrade. Other significant observations were that it had a high molecular weight and an unusually high melting point although it does not flow above this point.
The carbon-carbon bonds which form the spine of the polymer chain and the carbon-fluorine bonds are tremendously strong. The fluorine atoms form a consistent protective sheath over the chain of carbon atoms. This shields the polymer molecule from any attack by harsh chemicals. The fluorine atoms also reduce surface energy which results in the outstanding non stick properties and an exceptionally low coefficient of friction.
Fluoropolymers that are fully fluorinated, such as PTFE, PFA and FEP are almost inert to the harshest organic and inorganic chemicals as well as solvents over a very wide range of temperatures. Chemical inertness means that fluoropolymers that are fully fluorinated can be in contact with another substance, continuously without measureable chemical reaction or degradation occurring.
There are three fundamental causes of the almost universal chemical compatibility of fully fluorinated fluoropolymers:
PTFE (Polytetrafluoroethylene) is a synthetic fluoropolymer of tetrafluoroethylene. It is a solid form of fluorocarbon and a compound with elevated molecular weight. It consists entirely of fluorine and carbon atoms. It is hydrophobic, which means that it cannot be made wet by substances containing water or by water itself. This is possible due to fluorine’s high electro-negativity and low levels of friction coefficients against all types of solids. Although PTFE is considered to be a thermoplastic, it only softens at its melting temperature and does not melt-flow to the same extent as FEP and PFA. PTFE has a continuous operating temperature of 260⁰c, and can be used intermittently (for periods of up to 30 minutes) at up to 290⁰c. It has a melting point of approximately 327⁰c.
FEP (fluorinated ethylene propylene) has similar properties to PTFE but it is a melt processable fluoropolymer which forms a smooth, continuous film which is highly beneficial in the chemical processing industry. FEP also has outstanding release properties and glass-like finishes can be obtained which can be of particular value for rubber or elastomer moulding applications where cosmetic properties are important. FEP has a lower melting and in-use temperature than PTFE or PFA. Maximum operating temperatures are normally rated at 205⁰c.
PFA (perfluoralkoxy) also has similar properties to PTFE but like FEP, it is a melt processable fluoropolymer and has a maximum in-use temperature of 260°c. PFA is available with varying molecular weights, which can be specified as part of the coating application and is particularly relevant if permeation or abrasion are key factors.
